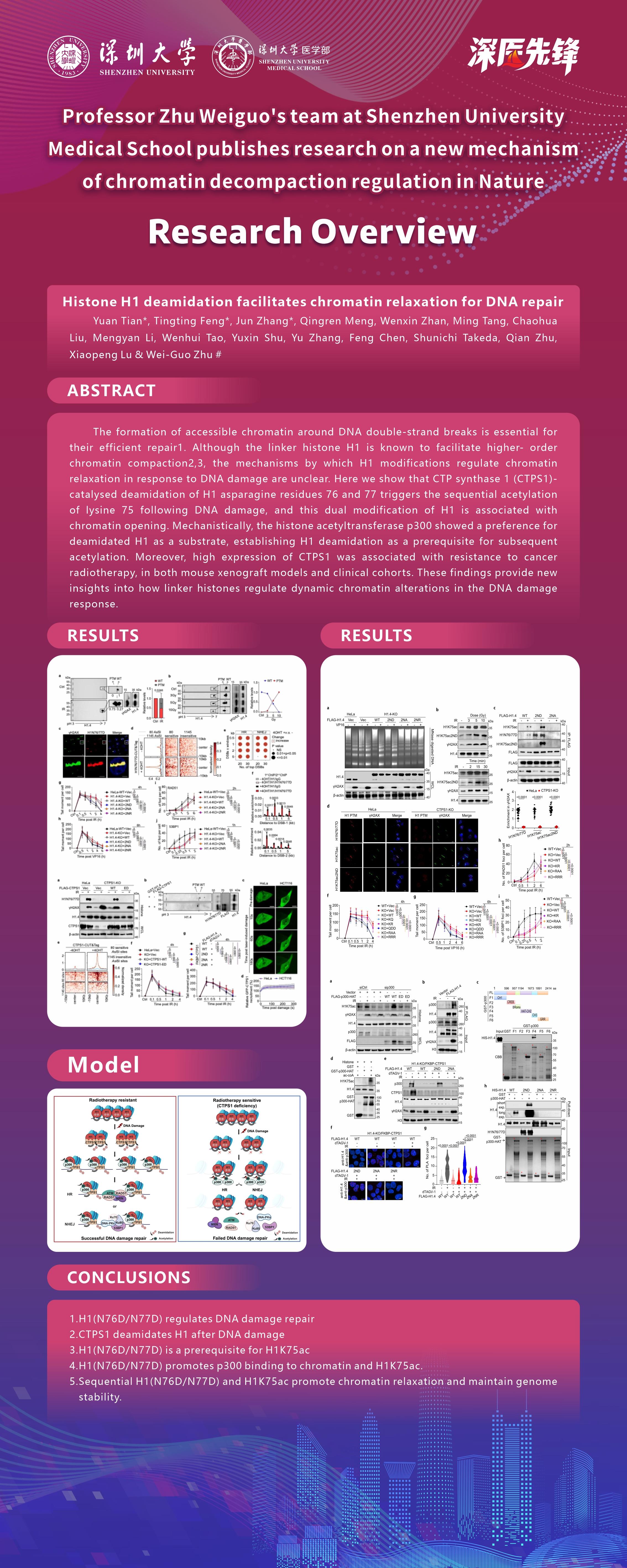
On April 16, 2025, Professor Zhu Weiguo's team from the School of Basic Medical Sciences of Shenzhen University Medical School and the Shenzhen University International Cancer Center published a groundbreaking research paper titled "Histone H1 deamidation facilitates chromatin relaxation for DNA repair" inNature. This research uncovers how the deamidation of histone H1 promotes chromatin relaxation and DNA damage repair, offering new insights into the design of precise therapeutic targets for cancer radiotherapy and chemotherapy. The study represents a major advancement in cancer prevention and treatment.

Cancer has become one of the leading causes of death worldwide, with China consistently reporting the highest number of new cancer cases globally. The primary treatments for cancer include surgery, radiotherapy, targeted therapies, and others. However, the five-year survival rate for cancer patients remains low. A major challenge in cancer treatment is the development of resistance to targeted therapies and radiotherapy. These therapies typically work by inducing DNA double-strand breaks in tumor cells, triggering cell death. However, if some tumor cells rapidly repair this DNA damage, they survive and continue proliferating, leading to resistance. Overcoming resistance to radiotherapy is one of the greatest challenges in cancer treatment today. Identifying critical DNA repair pathways and factors is therefore a key scientific focus. While global researchers primarily focus on the mechanisms of DNA repair following damage, there is still a limited understanding of the early cellular responses to DNA damage and the initiation of the repair process. One of the most crucial early events in DNA damage repair is the relaxation of DNA-enriched chromatin, which facilitates the exposure of broken DNA strands to repair factors, thereby enabling the repair process. Understanding the regulatory mechanisms of chromatin relaxation in response to DNA damage is of considerable scientific importance.
With strong support from Shenzhen University’s "2035 Pursuit of Excellence Research Plan" and the Medical School's "Organized Scientific Research Action Plan", Professor Zhu’s team has dedicated the past two decades to studying tumor epigenetic regulation and histone modification-mediated DNA damage responses, achieving a series of groundbreaking research outcomes. In recent years, the team has concentrated on the key early events in DNA damage response. On April 16, their findings were published online inNature, unveiling the mechanism by which deamidation of histone H1 promotes chromatin relaxation and facilitates DNA repair.
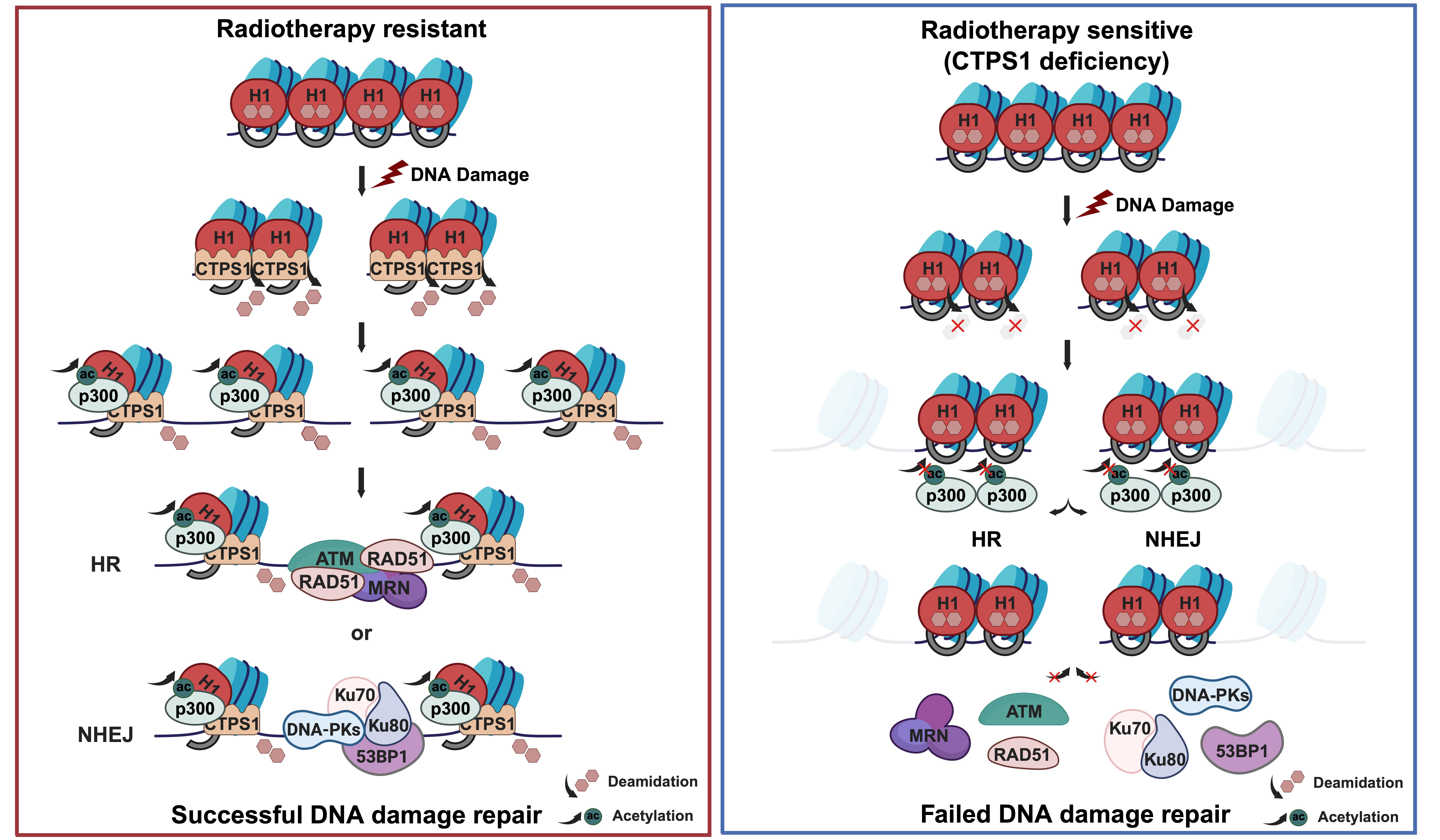
The study revealed that following DNA double-strand breaks, histone H1 undergoes specific deamidation at Asn76 and Asn77, catalyzed by CTPS1. This modification, in turn, promotes acetylation of the nearby Lys75 residue. The study also found that the histone acetyltransferase p300 demonstrates a strong substrate preference for deamidated H1, indicating that deamidation facilitates subsequent acetylation. Since Lys75 is located within the globular domain of histone H1, which plays a crucial role in DNA binding, acetylation at this site weakens H1's affinity for DNA, leading to the relaxation of nucleosomes and chromatin. This relaxation promotes the recruitment of key DNA repair proteins to the damage sites, facilitating efficient DNA repair. The study suggests that the specific "switch" formed by Lys75, Asn76, and Asn77 in histone H1 may regulate chromatin compaction and relaxation. This discovery provides detailed insights into the dynamic changes of chromatin under cellular stress and lays a robust theoretical foundation for the design of precise therapeutic targets in cancer radiotherapy and chemotherapy.
The corresponding author of the study is Professor Zhu Weiguo, Chair Professor at Shenzhen University and Director of the Shenzhen University International Cancer Center. The co-first authors include Tian Yuan, Assistant Professor at Shenzhen University, Feng Tingting, a PhD student in the cohort of 2022 at the Department of Biology, and Zhang Jun, Assistant Professor at Shenzhen University. In addition to the faculty and students from Shenzhen University, the study also involved collaborators such as Tang Ming from Tongji University’s Shanghai First Maternity and Infant Hospital, Liu Chaohua from Fudan University Shanghai Cancer Center, Shu Yuxin from Wannan Medical College, and Zhang Yu from the Department of Medical Genetics, Peking University Health Science Center.
Paper link: https://doi.org/10.1038/s41586-025-08835-0